
Incremental approaches are particularly valuable for packaged software (they already have a starting point) and for situations where final users experience difficulties expressing user requirements beforehand and in an abstract way.Ĭompanies should look for ways to reduce software verification effort according to GAMP 5 recommendations. During a sprint, design, development and testing people work closely together and produce a running increment of software that has already been tested.
#GAMP SOFTWARE CATEGORIES SERIES#
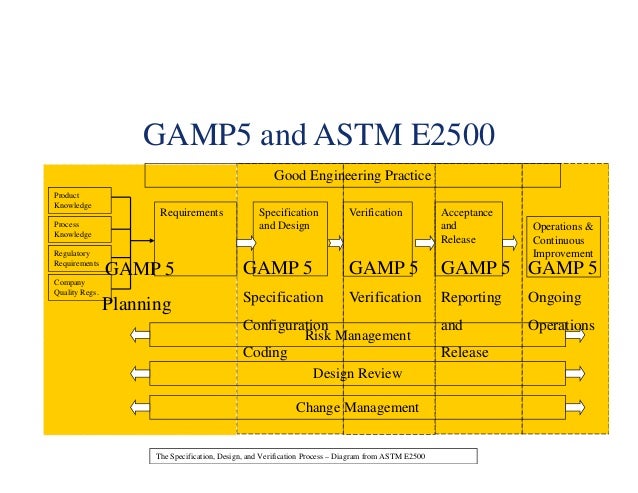
The idea is that in a series of consecutive “sprints”, a working and potentially releasable increment of product (software) is completed every 2 - 4 weeks. Latest approaches for software projects (like Rapid Prototyping or agile methods like Scrum) provide shorter cycle times and a more immediate look and feel of the software for the final user. The modelling of user requirements without seeing a running piece of software is abstract and usually requirements for modification arise when the final user deals with the running software for the first time. Vendors of software for regulated pharma environments maintain a state of the art quality assurance system and they have to prove that their core system is encoded and tested comprehensively.įigure 1: V-model or waterfall model There are two disadvantages of the traditional V-model: There are long cycle times from user requirement specification to user acceptance test and requirements may change in the meantime. It is questionable whether such an effort is constructive for configurable software packages (GAMP 5 category 4). As often seen in practice, OQ may take up to 50 % of the total effort outsourced for a project.
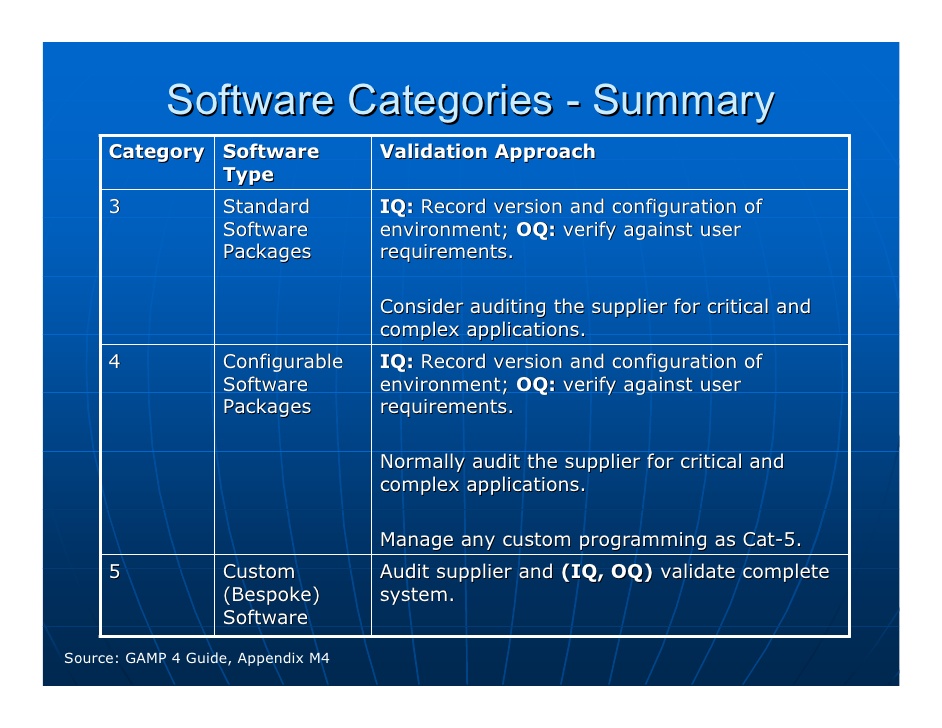
LIMS vendors provide their users with scripts based on which the technical functionality of the software in the clients’ (users’) environment (database, security, operating system, hardware platform, etc.) is tested. To help out their clients, LIMS vendors offer validation support mainly for “OQ” (OQ means “Operational Qualification”) for their clients (hereinafter called “users”). Verification is seen as a heavy burden in the implementation of Laboratory Information and Management Systems (LIMS). Formal software verification (often called validation) is a necessity in regulated pharma environments, as for example described and recommended in the GAMP 5 standard.


 0 kommentar(er)
0 kommentar(er)
